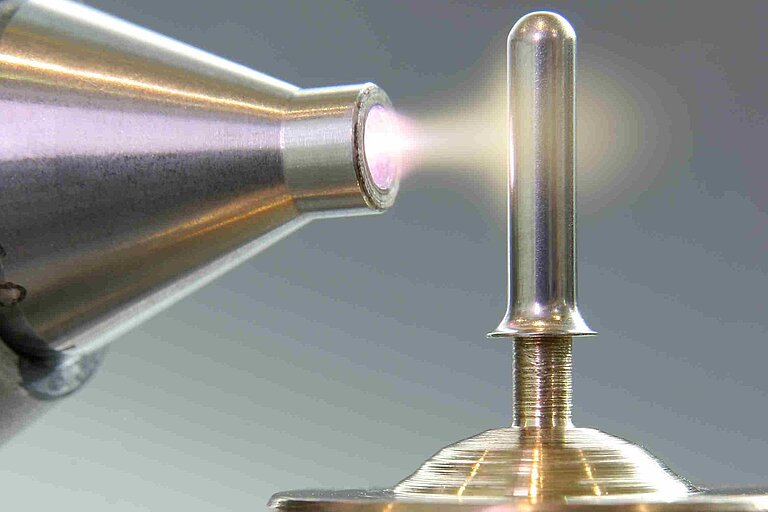
Plasma in Medical Technology
Manufacturing processes in medical technology demand the highest possible standards that far exceed the demands in most other industries. Surfaces must be not just clean but absolutely flawless or sterile. Beyond that, pre-treatment processes in medical technology must be very reliable and precisely reproducible. Plasma pre-treatment to improve adhesion of printing inks has been widely used in medical technology for some time. With Openair-Plasma®, Plasmatreat has taken it a big step further.
Clinically safe, sterile, reproducible—wide spectrum of applications for Plasma in medical technology
The Openair-Plasma® process allows for effective adhesion of hard/soft material combinations, applying finishes to membranes (filter media), and plasma functionalization of plastic surfaces. And all that is guaranteed to be aseptic thanks to plasma sterilization.
Advantages:
- Simple processes with dry, physical plasma treatment
- Reliable reproducibility of the pre-treatment parameters
- 100% process monitoring using PES (plasma emissions spectroscopy)
Plasmatreat has more than 30 years’ experience of using low-pressure plasma and atmospheric plasma to pretreat polymer surfaces, including treatment steps such as cleaning, adhesion promotion and deposition of functional coatings or layers. In our laboratories in Silicon Valley, we work with partners in industry to develop groundbreaking solutions.
Plasma modification. Casting medical filters.

The membrane on a medical filter must have optimal permeation characteristics for its intended purpose and be highly efficient. It is often manufactured from tufted capillaries. To ensure safe functioning of the membrane, there must be no punctures whatsoever. In particular, the membrane must be securely sealed to the filter’s end plates during manufacture.
Highly efficient manufacture of medical filters: safe casting of blood filters and oxygenators
Because of the hydrophobic nature of the membrane, reliable adhesion is only possible at that location with appropriate surface modification. In this particular medical technology application, Openair-Plasma® has already proven itself for years now—the membrane’s hydrophobic surface is pretreated at precisely the spot where the casting with the filter’s end plates is performed in the next step.
The result is a safe, high-quality processed medical filter.
Openair-Plasma® treatment of syringe needles
Needle hub bonding, print preparation and nano-coating
The barrels of disposable syringes and needle attachments, both mass-produced items, consist of non-polar plastics. This represents a challenge for further processing and packaging with respect to bonding, imprinting and functionalization of the syringes.
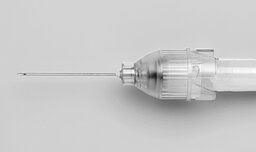

Openair-Plasma® treatment provides a very simple and versatile solution
- Secure adhesion: Selective pre-treatment of needle hubs ensures secure adhesion of syringes and needles.
- Optimal ink bonding: Prior to imprinting syringes, surface tension is increased using large-area plasma treatment of the non-polar plastic, which ensures secure adhesion of the printing ink.
- Functional nano-coating: Coating the interior of the syringe with a PlasmaPlus® process ensures low-friction functioning of the syringe plunger.
2C injection molding and encapsulation of inserts
Openair-Plasma® ensures impervious, secure bonds

When manufacturing medical devices, only a limited number of certified source materials may be used, so material combinations are complicated, in particular for 2C injection molding. Activation with Openair-Plasma® facilitates secure adhesion at the hard/soft interface, however, making it possible to nonetheless extrude non-compatible starting materials with each other.
A secure bond is also important when encapsulating inserts. In manufacturing inserts, for instance, the insert is placed in the die and it is encapsulated with polycarbonate. Pre-treatment with a potential-free, cycled Openair-Plasma® jet provides micro-fine cleaning of these metal inserts prior to injection molding.
The plasma energy simultaneously activates the surface, which ensures an absolutely impervious bond between the metal and the polycarbonate sheathing.
Interesting success stories in this field
Sediment-free plasma sealing
aseptic closure of glass vials

Sealed glass vials are considered the most secure containers for storing parenteral fluids. The vials are conventionally closed with a gas/oxygen mixture, whereby a propane/butane or natural gas is used as the burner gas. Depending on the adjustment of the gas mixture, this process can result in sediment and soot buildup in the inside of the vials, thus contaminating the contents. To address this problem, Plasmatreat has developed a special Openair-Plasma® jet technology with the highest plasma performance and 100% reproducible process parameters.
In this process, the plasma energy is targeted to achieve aseptic closure of the glass vials. Since the plasma does not burn in the conventional sense (combustion, oxidation), no exhaust or soot forms, and there is thus no contamination by flame reaction products.
Openair-Plasma® in laboratory technology
for optimal functional coating of plastics, hydrophilization of petri dishes

Germ formation and growth of a wide variety of microorganisms correlate specifically with oxygen content and wettability of surfaces. In laboratory medicine, that makes effective pre-treatment of surfaces of non-polar plastics especially important. The plasma process offers a range of application options for creating optimal surfaces for technical processes in the lab.
By treating petri dishes with Openair-Plasma®, surfaces can be hydrophilized. With the aid of the PlasmaPlus® process, an ultra-thin, glass-like nano-coating is applied. The variable specifiability of the plasma allows for targeted, controlled composition of the surface and thus individual adjustments for tissue engineering and cultivation of cell cultures.
Optimal wetting and flow behavior for lap-on-chip/biochip
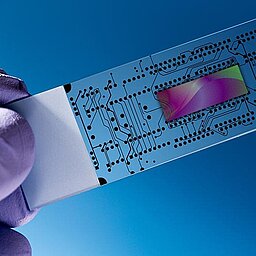
Microfluidic systems are new analytical methods for biotechnology and medical technology. On so-called BioChips (lab-on-chip), many analytical reactions can take place in parallel and therefore reliable information can be obtained in a very short time.
Microfluidics: Producing desired surface characteristics on lab-on-chip devices
The key to this type of analysis is the distribution and interaction of liquids on surfaces. The wetting and flow behavior on the carrier is decisive. Different carrier materials show different properties. Polymeric arrays of PS, PP, PMMA, PDMS or COC have as a rule a poorer wettability or react undesirably with the liquids.
Guided reengineering of surface characteristics thanks to Aurora low-pressure plasma
The surface properties of lab-on chips are positively altered with the Aurora low-pressure plasma: On the one hand, the surface wetting is enhanced in a controlled manner and, on the other hand, the interaction with the substrate is prevented through the deposition of a vitreous layer.
Hydrophobic layers with long-term stability
In addition, long-term stable, selective hydrophobic layers can be deposited on the surface using Aurora plasma. This effect allows the preservation of the microfluidic separation in some cases for several years.
Plasma pretreatment for reliable balloon catheters

In medical applications, catheters are used for minimally invasive treatment of conditions such as clogged arteries. The catheter is guided through the arteries until it reaches the spot requiring treatment. The blockage in the artery is then remedied with the help of a balloon. Various low-pressure plasma applications are available for manufacturing balloon catheters:
Reliable welding, sealing and bonding of catheter components
The delicate balloon must be reliably joined to the catheter tubing. This is normally done by laser welding. For optimal welding results, the catheter is pre-treated with low-pressure plasma. The plasma pre-treatment opens and cleans the surfaces of the different components to enable highly robust sealing. Many previously incompatible materials have been reliably welded in this way.
Drug-Coating
The balloons are coated with a drug in an exact dosage during the plasma process. The balloons are guided with the aid of the catheter to the affected area in the body. When the balloon is opened, the medication is released. The drug must adhere reliably to the balloon. At the appropriate place in the body, the drug must be dispensed safely in the targeted dosage.
Slip-Coating: Hydrophobic coating on outside surface of catheters
Friction between the catheter and blood vessel walls is often experienced as painful. A super-hydrophobic coating applied to the catheter not only reduces friction as the catheter is being introduced but also enables exact positioning of the balloon in the body.
For these applications, uniform pre-treatments under reproducible conditions is indispensable. This can be achieved via the plasma process of the catheter components. Other treatment methods, which include high temperatures or involving with electrical arcing can partially damage the catheter or balloon and pose risks to medical application.
The Aurora Tubing System developed by Plasmatreat delivers exceptionally consistent plasma intensity throughout the treatment space.
Thin-wall medical polymers are activated in high quality for safe welding. Functional coatings such as slip-coating or drug coating are applied homogeneously to the polymers using reactive gases in the low-pressure plasma process.